Abstract
Background: Autologous stem cell transplant (ASCT) is still a cornerstone in the treatment armamentarium of both Hodgkin's lymphoma (HL) and various non-Hodgkin lymphoma (NHL) subtypes. BEAM (carmustine, cytarabine, etoposide and melphalan) is the most commonly used conditioning regimen; however, due to limited availability and toxicity of carmustine, thiotepa-containing regimens have been suggested. We previously reported encouraging results with TECAM conditioning regimen (thiotepa, etoposide, cyclophosphamide, cytarabine and melphalan).
Objectives: We aimed to update our experience with TECAM as the conditioning regimen for ASCT in all lymphomas, by adding our experience from Oct 2013 to 2020 to the previously reported cohort of Jan 2000 - Sep 2013 (Grisariu et al 2018). Moreover, we aimed to utilize the detailed data on the two transplant cohorts, from different eras, to appreciate possible improvement in ASCT outcome in the recent era. Furthermore, we aimed to evaluate our ASCT results in different NHL subgroups.
Methods: We retrospectively analyzed all lymphoma patients who underwent ASCT in our center between 2000 and 2020.
Results: Three hundred and fifty-three patients were included (141 in the newer cohort added to the 212 in the cohort of 2000-2013 previously reported), all of whom were treated with TECAM, which is our standard conditioning regimen for lymphoma patients undergoing ASCT. The cohort included 126 HL patients, 108 diffuse large B-cell lymphoma (DLBCL) patients and 119 patients of other subtypes (among them 14 diagnosed with primary central nervous system lymphoma (PCNSL)). The median number of treatment lines before ASCT was 2 (range 1-7). Compared to the older cohort, the newer cohort was characterized by significantly poorer Eastern Cooperative Oncology Group performance status (ECOG-PS) prior to ASCT (45.6% vs 19.3% with ECOG-PS ≥1, p<0.001), while A Higher proportion of patients in the newer cohort were in complete remission (CR) status at transplantation (71.9% vs. 47.8%, p<0.001).
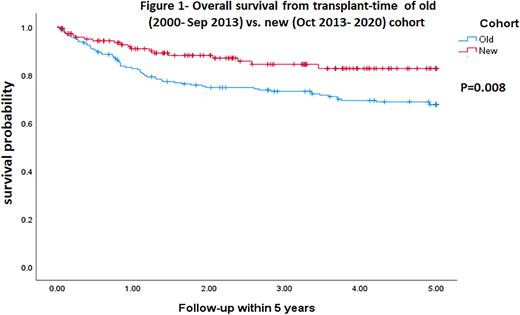
The median follow-up from ASCT was 38.8 months (range: 0.03-251.6). The 3-years progression-free survival (PFS) and overall survival (OS) rates post-ASCT were 59.8% and 79.3% respectively. For HL, the 3-years PFS and OS rates were 61.1% and 89% respectively; for DLBCL 59.4% and 67.9% respectively and for PCNSL 64.3% and 78.6% respectively. Outcomes of the newer cohort were significantly favorable (3-years OS of 87.3% vs. 73.8%, p=0.015, 5-year OS of 86.6% vs 69%, p=0.008, Figure 1). Poor disease status and poor ECOG-PS at ASCT entry were associated with worse outcomes across all lymphoma subtypes. Dose reduction was associated with a shorter PFS but did not affect OS. Similar to our previous report, patients entering transplantation for DLBCL with a partial response (PR) achieved similar outcomes as those with CR.
Eighteen patients died within the first 100 days, eight due to disease progression, and 10 due to transplant related causes (2.8%). There were no cases of interstitial pneumonitis syndrome (IPS). Twenty cases (5.7%) of secondary malignancies were documented.
Discussion: Our results confirms that TECAM is an effective and safe conditioning regimen for ASCT in patients with HL and various NHL's, including favorable results in PCNSL. Though outcomes were compared to published experience with BEAM, we have shown similar efficacy with a favorable toxicity profile. Despite a higher proportion of frail patients, the newer cohort's outcomes were favorable, probably due to better lymphoma control at transplant entry, which can be explained by more efficacious therapies pre- and post-transplant. In the DLBCL cohort, ECOG performance status was more prognostic than achieving a CR before ASCT, a finding that is relevant to the optimal selection of patients to different second-line options in the era of chimeric antigen receptor (CAR-T) availability.
Conclusion: TECAM is a safe, effective, CNS-penetrating conditioning regimen for ASCT, in HL and various NHL's.
Disclosures
Avni:MSD: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal